End to end handling of spontaneous reports, solicited
and clinical trial reports, medico legal cases etc from triage and database entry
including medical review.
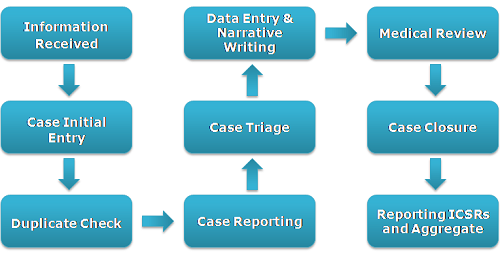
This service area covers the processing of individual case safety reports (ICSRs)
originating from various sources:
- Post-marketing non-solicited/Spontaneous reports
- Clinical reports
- Special reports (Medico-legal and Literature)
- Related services
Data from source documents sent by clients and business partners is processed (data
entry and MedDRA coding) in to a Drug Safety/Pharmacovigilance database (Arisg ,
Argus etc.) after a duplicate search, on behalf of the clients. This may involve
an initial triage and a subsequent medical review/assessment of the report by physicians.
Once finalized, the case reports are submitted to the regulatory authorities/business
partners.